Cold Boil | Hū Mātao
Introduction
Abstract
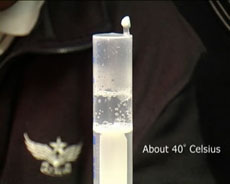
A syringe is used to make water boil near room temperature.
Principles Illustrated
Water boils at 100 °C at one atmosphere of pressure but at lower temperatures under lower pressures. Water will actually boil at room temperature if the pressure is low enough. Here we make water boil at room temperature, or more vigorously at about 40 °C, by pulling back the handle of a syringe filled with water. We can understand this as follows. the water molecules have varying amounts of energy, and some are able to leave the liquid and enter the vapour. When we reduce the pressure above the liquid by pulling down the handle of the syringe, we make it easier for water molecules to leave the liquid, thus reducing the boiling temperature.
Content
Video
English version
Te Reo Māori Version
Instructions
Fill the syringe to around one quarter to one third full from a bucket of room temperature or warm water and seal the top (we usually use Blu-Tack). First pull the syringe back and wait for dissolved air to come out of the water and then return the piston to its original position gradually. Unseal the top and expel the small air bubble that forms. Now the syringe is ready for student use. Have a student pull down the plunger of the syringe to cause boiling. Mounting the syringe in a stand can make it easier for younger students to pull the plunger and also for other students to see the boiling, particularly if larger syringes are used, but have students wear goggles (see safety notes).
There is a neat bit of more advanced physics here as well. If you simply partly fill the syringe with water from a bucket, expel as much of the air as possible, and pull down the syringe plunger as described above you will see boiling that is nucleated on the inside surfaces of the syringe. This is seen in our video. It looks a lot like water boiling in a glass kettle with large bubbles. Use about 20 ml of water in a 50-60 ml syringe or about 5 ml in a 20 ml syringe. But you can also see a rather different effect. If you seal a SMALL (20 ml perhaps) syringe with about 5 ml of water, pull the plunger down far and release suddenly, expel the air, and then reseal and pull it slowly you can see boiling throughout the liquid with lots of small bubbles. This effect is discussed well on the Exploratorium website: http://www.exploratorium.edu/mars/coldboiling.php. A large syringe is more likely to shatter when released suddenly. We are in the process of making a video that shows these two experiments side-by-side.



Other Information
Safety
Have the students wear goggles, particularly with larger syringes. The top of the syringe can shatter if the handle is released suddenly! This is less likely to happen with the 20 ml syringes.
Individual teachers are responsible for safety in their own classes. Even familiar demonstrations should be practised and safety-checked by individual teachers before they are used in a classroom.
Related Resources
Te Whakamātao Mā te Paera (Cooling by boiling), Werawera Mātao (Cold Sweat), Pena_i_te_Matao_o_Tangaroa (Solid Nitrogen)
Credits
This teaching resource was developed by the Te Reo Māori Physics Project with support from
- Te Puni Kōkiri
- The MacDiarmid Institute
- Faculty of Science, Victoria University of Wellington
- School of Chemical and Physical Sciences, Victoria University of Wellington
- The New Zealand map shown on the poster frame above is used with permission from www.nz.com.
- The storm image is from NASA , Courtesy NASA/JPL-Caltech This teaching resource was developed in collaboration with Mabel Stewart, a New Zealand Science, Mathematics and Technology Teacher Fellow, 2008, hosted by Victoria University School of Chemical and Physical Sciences. When she worked on this project, Mabel taught at Bishop Viard College in Porirua, New Zealand. See NZSMT Teacher Fellowships for more information about the Teacher Fellow Programme.
- The photograph of Taranaki is from the Natural Sciences Image Library
- The Mount Everest photos are from the Royal Geographic Society